Abstract
Introduction:
We have demonstrated peripheral blood (PB) blasts negatively affected chimeric antigen receptor T cells targeting CD19 (CART19) production and the clinical efficacy of CART19 therapy in patients (pts) with refractory/relapsed B cell acute lymphoblastic leukemia (r/r B-ALL) (published in Molecular Therapy: Methods & Clinical Development ,2021). Whether sorting T cells before activation might be helpful for ex-vivo CART19 quality and in-vivo treatment efficacy requires further investigation. Here, we retrospectively analyzed 40 pts with or without upfront T cell purification to evaluate these indexes.
Patients and Methods:
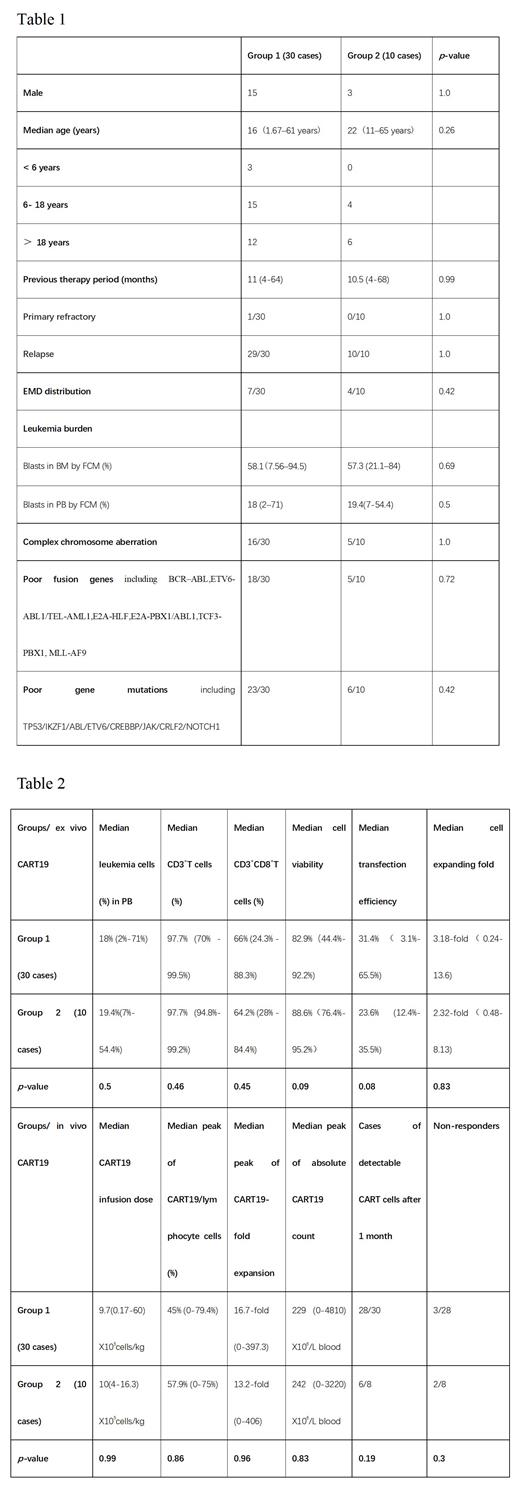
From July 2021 to June 2023, 40 r/r B-ALL with PB blasts at time of apheresis experienced CART19 therapy. After leukapheresis, all pts' PBMCs were isolated using lymphocyte separation liquid. 30 pts didn't perform T cells selection (group 1) and 10 pts performed T cells selection (group 2). All of cells were activated with CD3 and CD28 antibodies for 24h, then transduced with the lentivirus encoding anti-CD19-CD3zeta-4-1BB CAR and cultured for 5-6 days in serum-free media containing IL2, IL7, IL15, IL21. The baseline characteristics, including age, sex, previous therapy period, blasts in BM, EMD distribution, complex chromosome aberration, poor fusion genes and gene mutations, did not differ between the two groups (Table 1). Cell viability, expanding fold, percentage of CD3 +T and CD3 + CD8 +T cells, transfection efficiency, B cells were all examined pre CART19 infusion. All pts received cyclophosphamide 250 mg/m2/d X 3d, and fludarabine 30 mg/m2/d X 3d, followed by CART19 infusion with a median number of 9.7(0.17-60) X10 5cells/kg in group 1 and 10 (4-16.3) X10 5cells/kg in group 2 ( P=0.99). PB and BM blasts was determined by flow cytometry (FCM). All pts underwent bone marrow (BM) biopsy examination and FCM studies on day 15 or day 30 to determine the response and remission status.
Results:
At time of apheresis, group 1 and group 2 had a median leukemia cell of 18% (2%-71%) and 19.4% (7%-54.4%) in PB ( P=0.5), respectively. In group 1 and group 2, the median percent of CD3 +T and CD3 + CD8 +T cells were 97.7% (70% - 99.5%) vs. 97.7% (94.8%-99.2%) ( P=0.46) and 66% (24.3% - 88.3%) vs. 64.2% (28% - 84.4%) ( P=0.45), respectively. The median cell viability, transfection efficiency and cell expanding fold in the final products was 82.9%(44.4%-92.2%) vs. 88.6%(76.4%-95.2%)( P=0.09), 31.4%(3.1%-65.5%) vs. 23.6% (12.4%-35.5%) ( P=0.08) and 3.18-fold(0.24-13.6) vs. 2.32-fold(0.48-8.13) ( P=0.83), respectively. Both of normal B and leukemia cells were undetectable pre CART19 infusion in two groups. After CART19 infusion, the median peak CART19/ lymphocyte cells, expanding fold and absolute CART19 count in vivo was 45% (0-79.4%) vs. 57.9% (0-75%) ( P=0.86), 16.7-fold (0-397.3) vs.13.2-fold (0-406)( P=0.96) and 229 (0-4810) X10 6/L blood vs. 242 (0-3220) X10 6/L blood ( P=0.83) in two groups, respectively. In group 1, 28/30 cases were evaluable and 25/28 (89.3%) obtained complete remission (CR)/ incomplete count recovery CR (CRi) and 24/28 (85.7%) obtained minimal residual disease (MRD) - CR. 1/2 patient unevaluable died from pneumonia due to severe and persisted neutropenia. One no-responder was infused at <1 X10 5 cells/kg and his culture failed to expand in vivo, and the other no-responder was infused at 1.16 X10 5 cells/kg (with TEL-AML1 positive). In group 2, 8/10 cases were evaluable and 6/8 (75%) obtained CR/CRi with all MRD - CR (75%). The two non-responders had no detectable CART19 in PB after infusion for one month. Results were shown in Table2.
Conclusion:
We have demonstrated pts with PB blasts without sorting T cells had poorer ex-vivo CART19 quality and in-vivo treatment efficacy compared to pts without PB blasts (published). Sorting T cells before activation is expected to remove the adverse culture conditions in the system. However, in the present study, upfront T cell purification didn't improve ex vivo cell characteristics and in vivo cell dynamics in r/r B-ALL pts with PB blasts, showing that the negative influence of PB blasts at the time of apheresis could not be reversed by upfront T cell purification. Therefore, leukemia burden in PB is suggested to be reduced before leukapheresis. Absolutely, one of the limitations of our study is that a relatively small number of products was evaluated, and larger samples need to be included to further demonstrated.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal